Phase II - Cluster Randomized Clinical Trial
An important part of the IMMERSE project is the cluster randomized clinical trial, a study to evaluate the added value of the DMMH in mental health care in 4 European countries: Belgium, Germany, Scotland, and Slovakia. In total, 8 clinical sites participate, including 3 units per clinical site. For each country, the aim is to include 108 participants by May 2023.
What does the study look like?
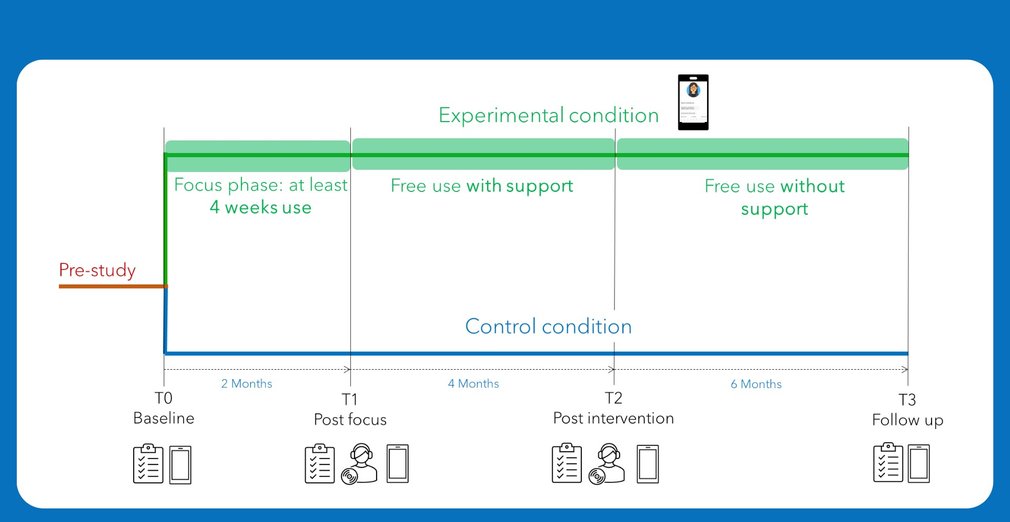
At the beginning of our clinical study, the clinical units are randomly assigned to a condition: the experimental condition or the control condition. The units that are in the experimental condition will test the DMMH tool in practice, whereas the units in the control condition will not use the DMMH tool. A control condition is necessary to evaluate the added value of the DMMH to induce changes in clinical outcomes as compared to care as usual. All participants, regardless of the condition, will complete questionnaires at the start of the study (T0), but also after 2 months (T1), after 6 months (T2), and after one year (T3). In the experimental condition, Individuals will do 6 days of monitoring in addition to the questionnaires. These time points already indicate that the trial will take one year for each participant, however, this does not mean that the participant is actively involved for the entire year.
1. The individuals who are in the experimental condition will go through 3 phases:
The focus phase.
During the focus phase, participants use the DMMH system for at least 4 weeks. After each week, the participant and the therapist discuss the gathered data as part of a therapy session. Looking at this data can help the participant to get more self-insight, but this also gives the therapist a glimpse of the participant’s daily life outside the hospital or therapy session. At the end of this phase, the research team will interview some participants to learn about their experiences with the DMMH system.
The phase of free use with support of the research team.
During this phase, the participant and their therapist can decide when and how they want to use the DMMH system. The research team will provide support in this phase, in case of technical issues, questions, etc. At the end of this phase, the research team will interview some participants to learn about their experiences with the DMMH system.
The phase of free use without support of the research team.
The only difference with the previous phase, is that the research team won’t be present anymore to provide support.
2. The participant in the control condition will only be contacted for the questionnaires at time points T0, T1, T2, and T3.